Universal Medica Group is a key partner to the life sciences and healthcare industries, structured around several specialized subsidiaries. Each entity brings unique and complementary expertise to support pharmaceutical companies, biotech firms, medical device manufacturers, and other health stakeholders throughout the lifecycle of their products.
This structure enables us to offer tailored, innovative solutions that meet regulatory requirements, ensuring the quality, safety, and performance of healthcare products on the market.

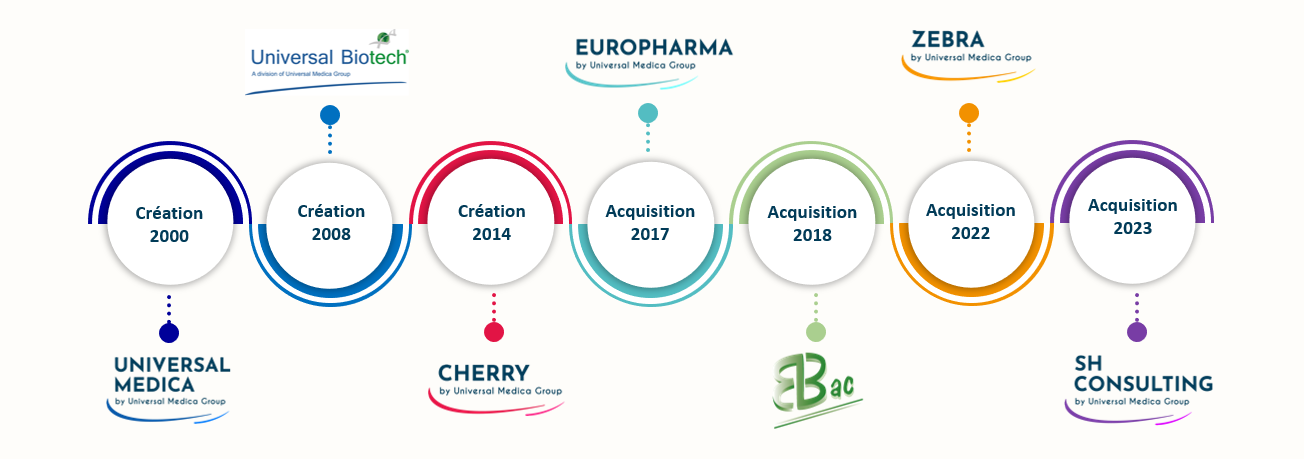
Universal Medica: Expertise Serving Compliance and Safety
For over 20 years, Universal Medica has supported life sciences and healthcare industries players by offering specialized services in:
- Medical Information: handling inquiries from healthcare professionals and patients, ensuring traceability and documentation of exchanges
- Pharmacovigilance and Health Vigilance: monitoring adverse effects, managing risk plans, and ensuring regulatory compliance
- Regulatory Affairs: supporting the registration of medicines and medical devices, submission of dossiers, and lifecycle management
- Compliance: ensuring transparency obligations are met and governing relationships between industry stakeholders and healthcare professionals
Why Choose Universal Medica?
With its scientific and regulatory expertise, Universal Medica guarantees clients rigorous and compliant management of legal and scientific obligations, ensuring patient safety and product reliability in the market.
Europharma: Excellence in Training and Skills Development
Europharma is a leading provider of specialized training for client teams, offering expertise and tailored support.
- Customized Programs: training in medical information, regulatory affairs, compliance, pharmacovigilance…
- E-learning and In-Person Training: via a dedicated digital platform and on-site sessions
- Training Modules Aligned with Laboratory Needs: skill development, regulatory mastery, and optimization of business practices
Why Choose Europharma?
With over 30 years of experience and Qualiopi certification, Europharma supports the healthcare and Life Sciences industries in workforce development, ensuring up-to-date expertise aligned with sector evolutions.
SH CONSULTING: The Expert in Supporting Pharmaceutical Industrial Sites
SH CONSULTING supports pharmaceutical and biotech manufacturing sites with tailored solutions to ensure GMP compliance and effective project management.
- Industrial project management and compliance of equipment and processes
- Preparation for health agency inspections
- Quality Assurance (QA) monitoring: Product, Supplier, and System
- Supplier audits, specification drafting, and complaint tracking
- Strategic sourcing and support to ensure operational efficiency
Why Choose SH CONSULTING?
With an expert, flexible, and personalized approach, SH CONSULTING supports industrial players at every key stage of their projects, working closely with on-site teams. Our mission: to ensure compliance, quality, and sustainable performance of pharmaceutical industrial sites.
CHERRY ZEBRA AGENCY: the health communications agency integrated into the Universal Medica Group
CHERRY ZEBRA AGENCY is the communications agency created from the merger between CHERRY and ZEBRA in November 2025.
Our areas of expertise:
- COMMUNICATION STRATEGY
- MEDICAL EDUCATION
- MEDICAL CONTENT MANAGEMENT
- DIGITAL
- HEALTH MEDIA PUBLISHERS
- OFFICINE PLUS
Our deliverables:
NON PROMOTIONAL COMMUNICATION
- Slide kit
- Kakemono
- Newsletter
- Scientific brochure
- Email campaigns
- Website
- Escape game
- Web banner
- Motion design
PROMOTIONAL COMMUNICATION
- Sales Aid
- Dosing information sheet
- Totem
- Press Ad
- Email campaigns
- Web banner
- Videos
MEDICAL EDUCATION
- Webinar
- KOL advisory board
- Event
- Videos
- Invitation cards
